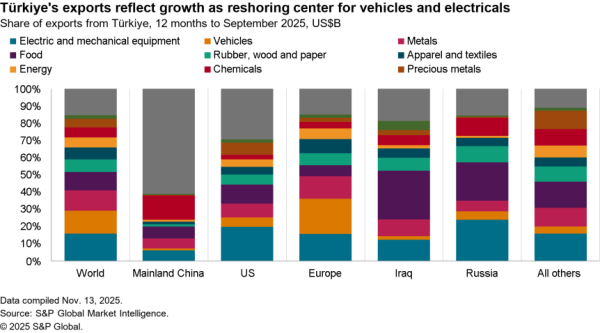
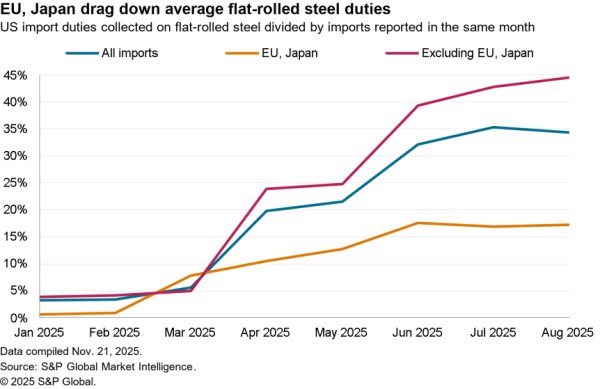
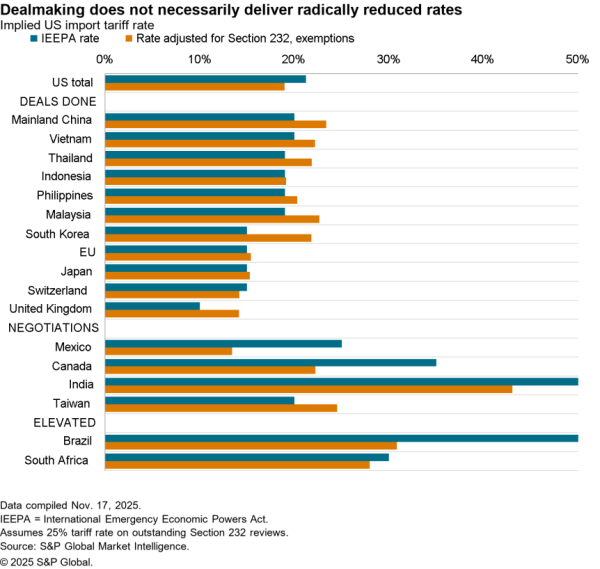
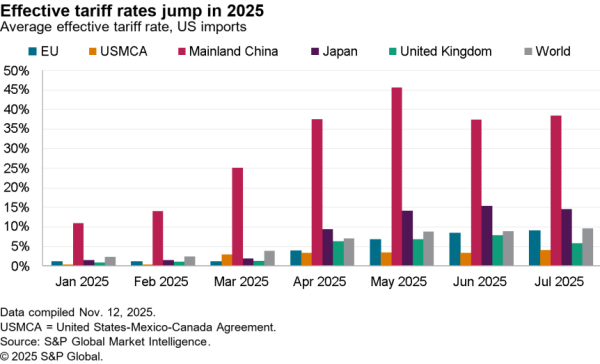
The U.S.-South Korea Partnership Agreement signed by U.S. President Joe Biden and South Korea President Jae-In includes a wide range of commitments on critical supply chains, as flagged in Panjiva’s research of May 24.
In healthcare there were commitments to use the KORUS trade deal, negotiated by the Trump administration, as a mechanism for cooperating to “greatly scaling up global COVID-19 vaccine supply” including a statement that “Korea will provide expanded production capacity at manufacturing facilities in Korea“. The need to scale up vaccine production follows the decision by Serum Institute to pause exports until year end.
South Korea may also be looking to build up its own vaccine production / export business. Thus far South Korea has produced predominantly for domestic manufacturing. Indeed, Panjiva’s data shows South Korea represented just 0.5% of global vaccine exports in 2019. That may change as new facilities are rolled out, with Samsung Biologics having agreed a deal to bottle Moderna’s vaccine in South Korea, Yonhap reports.

Source: Panjiva
Notably the Biden-Moon meeting also flagged plans being put in place to “prepare for and mitigate the damage of the next biological threat” by initiating “a new partnership with other like minded partners to establish a multilateral, sustainable and catalytic health security financing mechanism this year“. While nebulous now this moves the debate beyond the relaxation, or otherwise, of intellectual property relating to COVID-19 vaccines.
A focus on the long-term is important given the extensive timeframe needed to rollout supply chains in vaccine production, particularly including an investment in workforce as well as physical infrastructure.
In the near-term momentum is building behind a globally coordinated push to boost vaccine production. The G20 Rome Declaration is helpful, as are commitments from producers to supply 1.3 billion doses for emerging markets in 2021 as reported by Associated Press. As with climate change though, nation-state detailed commitments matter more than global aspirations.
The U.S. will remain central to ensuring a steady flow of exports of pharmaceutical ingredients. Panjiva’s data shows shipments from the U.S. to South Korea in Q1’21 inched just 0.9% higher year over year but have been growing over the longer term with a 35.8% increase versus Q1’19, while representing just 2.8% of total shipments.
That came against the backdrop of a 12.2% rise in total U.S. exports in Q1’21 including a 14.9% year over year rise in shipments to Mexico and Canada as well as a 13.5% rise in exports to Europe.

Source: Panjiva