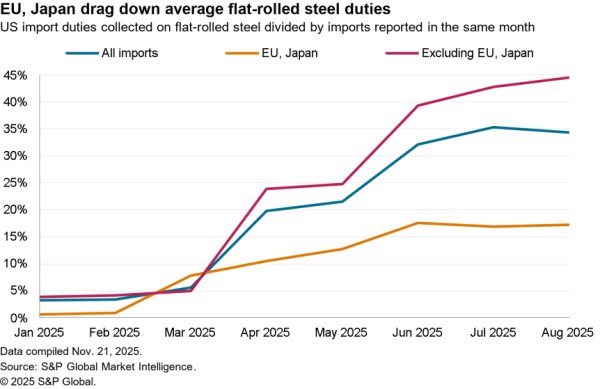
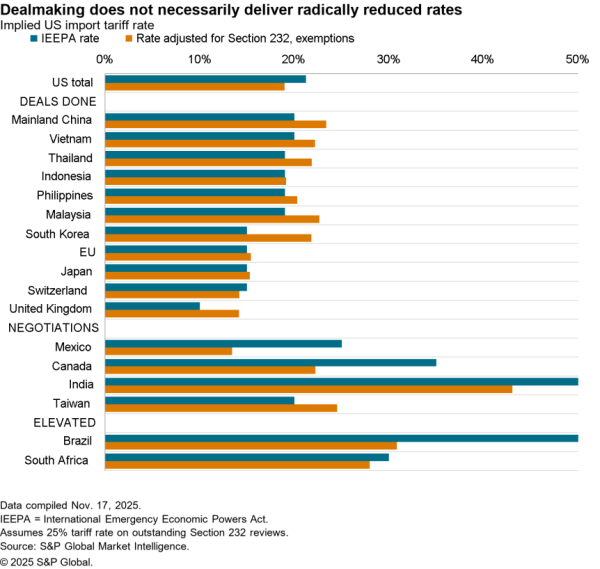
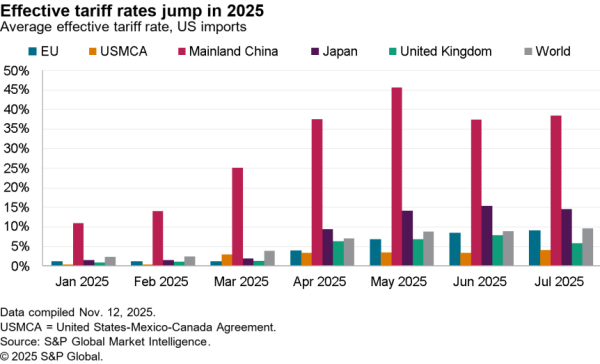
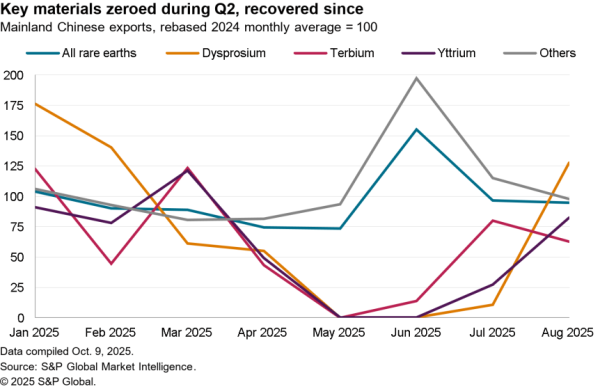
The Indian government has removed restrictions on the export of precursors for acetaminophen / paracetamol with immediate effect. The restrictions had been put in place as part of a wide range of medical supply measures in early March. India, like the EU as discussed in Panjiva’s May 28 report, has begun to roll the measures back as medical supply chains stabilize and as COVID-19 has begun to abate in places.
Yet, the earlier restrictions meant that Indian exports had fallen, shown for example by a 22.1% year over year slide in shipments to the U.S. in the first half of May, Panjiva’s data shows. That included a 20.7% decline in shipments of packaged drugs and a 36.0% slump in shipments of precursors.
The latter will have caused damage to U.S. drug manufacturing and may cause hold-ups as U.S. factories return to operation in May. There had already been a more extensive downturn in precursors, which had also dropped in February through April.

Source: Panjiva
Among the major Indian drug suppliers, the downturn in shipments in April was fastest in shipments linked to Alkem and Lupin which declined by 73.3% and 72.7% respectively. Shipments associated with Cadila and Intas meanwhile actually continued the double digit pace of growth seen in the prior six months with improvements of 54.4% and 14.3% respectively.

Source: Panjiva